In section 2.2.3 we concluded that the free volume available to a rod is independent of fugacity. Therefore the complete phase diagram for the needle-sphere mixtures including the fluid-solid transitions can be obtained using first-order perturbation theory. The coexistence densities for the solid-liquid and the solid-gas transition are easily calculated from the chemical potentials and the pressures in eqn. 2.14-2.15. The resulting phase diagrams for different rod lengths are shown in figure 2.4. The most obvious result is that a stable liquid-gas two-phase region is indeed possible. If the rods get smaller than 0.3
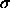 the vapor-liquid phase separation becomes metastable. One can imagine that at this point the rods start to fit in the interstitial spaces of the solid phase. This causes the free energy of the solid vapor equilibrium to be lower than that of the liquid vapor equilibrium.
the vapor-liquid phase separation becomes metastable. One can imagine that at this point the rods start to fit in the interstitial spaces of the solid phase. This causes the free energy of the solid vapor equilibrium to be lower than that of the liquid vapor equilibrium.As discussed above the coexistence curves shift to lower fugacities with increasing rod length. The interaction range between the particles becomes larger and the necessary depth of the effective potential to induce phase separation which is governed by the rod fugacity is smaller. The fluid-solid coexistence region becomes wider at high fugacity. This is a common phenomenon in fluid-solid transitions. If the fugacity increases, the free energy in the fluid decreases more than the free energy of the solid. To compensate this, the equilibrium solid phase has to become denser and the fluid phase more dilute.
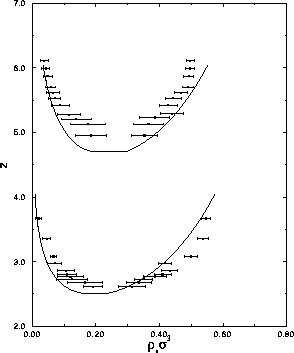
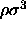 .
The upper curve and points correspond to
.
The upper curve and points correspond to 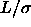 =2, the lower to
=2, the lower to
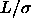 =3.
=3. 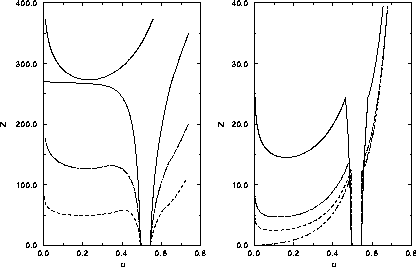
 . The lines correspond to the phase
boundaries and are obtained by first-order perturbation theory as
given in the text. Left picture: solid line
. The lines correspond to the phase
boundaries and are obtained by first-order perturbation theory as
given in the text. Left picture: solid line 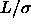 =0.2, dotted line
=0.2, dotted line
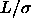 =0.3, dashed line
=0.3, dashed line 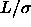 =0.5. Right picture: solid line
=0.5. Right picture: solid line 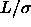 =1, dotted
line
=1, dotted
line 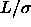 =2, dashed line
=2, dashed line 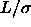 =3 and dot dashed line
=3 and dot dashed line
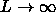 . The solid curve in the left picture is the metastable fluid-fluid binodal for
. The solid curve in the left picture is the metastable fluid-fluid binodal for 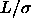 =0.2.
=0.2.