Peptide aggregation
By Marieke Schor
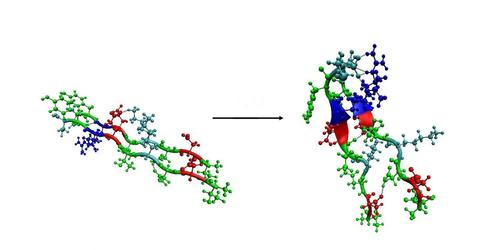
Aggregation or, more specific, fibril formation is a very common process in nature. On the one hand you could think of functional fibrils like microtubules or gossamer. However, fibrils are also associated with diseases like Alzheimer’s and type II diabetes. The most popular model for fibrillar assembly is the nucleation-polymerisation model. First a stable nucleus has to be formed as the rate limiting step. After this, further elongation of the fibril is very fast. So far it is not really known what drives these unstructured peptides to form highly ordered aggregates with a high beta-sheet character but it seems to be a very common feature among proteins. It is thought that hydrogen bond formation and hydrophobic interactions are the main driving forces for aggregation. At the moment we work on two different fibril forming sets of peptides. For these systems we want to investigate the mechanism behind both nucleus formation and further elongation of the fibril. For this we use molecular dynamics (MD) simulations and parallel tempering techniques (hier link naar stukje Jocelyne) As fibrils rapidly become to large to study with standard MD simulations once the first steps are clear, we will use coarser and coarser models to follow fibril growth at time and length scales of interest.
Silk-based block co-polymers
(In collaboration with the Laboratory of Physical Chemistry and Colloid Science, Wageningen Stimulus-responsive, nanostructured, self-assembling polymer materials have potential applications in tissue-engineering, nanotechnology and materials science. Silk-based block co-polymers consist of two silk-based blocks (S) and two collagen-like blocks (C), with the S blocks either on the outside (SCCS) or on the inside (CSSC). Upon changing to a lower pH the glutamate (E) residues become protonated and the protein starts forming very regular fibrils. Once the fibril is formed the silk blocks of the proteins have a regular beta-sheet structure while in solution the structure is unclear.
Amyloid forming peptides
(In collaboration with the Cell Biology and Histology group of the AMC) Usually defective or damaged proteins are rapidly degraded by proteases to avoid negative effects on cell function. However, if this fails misfolded and protease-resistant protein fragments can form aggregates in the cell as is commonly seen in a number of neurodegenerative diseases. The nine amino acid model peptide that we use shows rapid aggregation both in fluorescence microscopy experiments and in our simulations.
