


Next: Phase diagram for Up: Brief summary of Previous: Brief summary of



Next: Phase diagram for
Up: Brief summary of
Previous: Brief summary of
Figure 5.1 shows the computed phase diagram of
hard-spherocylinders in the region between 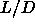 =0 (hard spheres) and
=0 (hard spheres) and
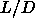 =5. The black squares indicate the reduced transition densities
for
=5. The black squares indicate the reduced transition densities
for 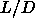 values at which simulations were performed.
In this and following figures, the reduced density
values at which simulations were performed.
In this and following figures, the reduced density 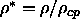 is the density relative to the density of regular
close packing of spherocylinders:
is the density relative to the density of regular
close packing of spherocylinders:
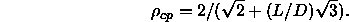
For particles with 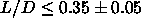 , the
isotropic fluid freezes to form a plastic crystal (rotator phase). At
higher densities, the rotator phase undergoes a first-order transition
to the orientationally ordered phase. As
, the
isotropic fluid freezes to form a plastic crystal (rotator phase). At
higher densities, the rotator phase undergoes a first-order transition
to the orientationally ordered phase. As 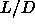 is lowered to zero,
this transition moves towards the density of regular close packing
is lowered to zero,
this transition moves towards the density of regular close packing 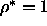 .
Between
.
Between 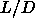 =0.35 and
=0.35 and 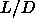 =3.1, only two phases occur: the
low-density isotropic phase and the high-density, orientationally
ordered, crystal phase. The smectic phase first becomes stable at the
I-SmA-S triple-point which is located
=3.1, only two phases occur: the
low-density isotropic phase and the high-density, orientationally
ordered, crystal phase. The smectic phase first becomes stable at the
I-SmA-S triple-point which is located
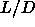 =3.1. The nematic phase becomes stable at
=3.1. The nematic phase becomes stable at 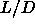 =3.5. The
nematic-smectic transition takes place around
=3.5. The
nematic-smectic transition takes place around 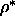 =0.5 and is initially clearly first
order, but the density jump at the N-S transition shrinks
with increasing
=0.5 and is initially clearly first
order, but the density jump at the N-S transition shrinks
with increasing 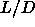 .
The smectic to solid transition is located at
.
The smectic to solid transition is located at 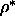 =0.66--0.68 and
is also first order.
=0.66--0.68 and
is also first order.