
Evaluation of the hard sphere fluid-solid coexistence line as a function of polydispersity can be done by application of the Gibbs-Duhem integration method recently developed by Kofke [84]. In this method two phases are simulated simultaneously at the same state conditions. The technique allows a series of simulations to trace a line of coexistence in the plane of two state variables; for the purpose of describing the method let us say that these variables are the temperature and pressure, respectively. Chemical potential equality between the phases is ensured by starting the process with two known equilibrium phases, and subsequently applying thermodynamic integration to select the appropriate pressure while the temperature is varied from one simulation to the next in the series. The integration path may be derived from the Gibbs-Duhem equation
where 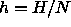 and
and 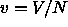 are the molar enthalpy and volume,
respectively. For two coexisting phases to remain in equilibrium when
the temperature is changed, the pressure must vary in a way that
maintains chemical potential equality between them. The required
change can be derived from eqn.
4.6
are the molar enthalpy and volume,
respectively. For two coexisting phases to remain in equilibrium when
the temperature is changed, the pressure must vary in a way that
maintains chemical potential equality between them. The required
change can be derived from eqn.
4.6
where 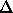 indicates a difference between the two phases. Eqn
4.7 is known as the Clapeyron equation. It is a simple first
order differential equation which can be integrated using a
predictor-corrector scheme. The 'initial condition' is a known point
at the coexistence line in the T,P plane. By simulating the two
coexisting phases simultaneously at the equilibrium pressure and
evaluating the right hand side of eqn.
4.7, one can predict
the pressure at another temperature not far away. Simulation at this
P and T yields new values of
indicates a difference between the two phases. Eqn
4.7 is known as the Clapeyron equation. It is a simple first
order differential equation which can be integrated using a
predictor-corrector scheme. The 'initial condition' is a known point
at the coexistence line in the T,P plane. By simulating the two
coexisting phases simultaneously at the equilibrium pressure and
evaluating the right hand side of eqn.
4.7, one can predict
the pressure at another temperature not far away. Simulation at this
P and T yields new values of 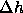 and
and 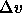 , which can
be used to correct the predicted pressure while the simulation
continues to proceed. The process is then repeated to get the next
, which can
be used to correct the predicted pressure while the simulation
continues to proceed. The process is then repeated to get the next
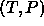 coexistence state point. Details of the method may be found
elsewhere [84,88].
coexistence state point. Details of the method may be found
elsewhere [84,88].
In the case of polydisperse hard spheres we do not need the
temperature as an independent variable, but instead we need a measure
for the polydispersity. An obvious choice is  , as it occurs in
the fundamental equation 4.4. The Gibbs-Duhem equation for
polydisperse mixtures can be derived by combining
, as it occurs in
the fundamental equation 4.4. The Gibbs-Duhem equation for
polydisperse mixtures can be derived by combining 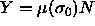 with eqn.
4.2
with eqn.
4.2
Using the same procedure as for the derivation of eqn. 4.4 we obtain
To ensure phase equilibrium, 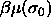 and the chemical
potential difference function given in eqn.
4.3 must be
the same in the two phases. The latter requirement is fulfilled simply
by using the same
and the chemical
potential difference function given in eqn.
4.3 must be
the same in the two phases. The latter requirement is fulfilled simply
by using the same  (and
(and 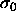 ) in both phases. The first
requirement results in a Clapeyron type of equation which can be
derived from eqn.
4.9 by equating the right-hand side for
both phases and applying
) in both phases. The first
requirement results in a Clapeyron type of equation which can be
derived from eqn.
4.9 by equating the right-hand side for
both phases and applying 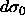 =0 and
=0 and 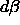 =0.
=0.
We can integrate in the 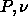 plane by measuring the second moment
of the composition distribution
plane by measuring the second moment
of the composition distribution 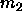 and the molar volume v in both
phases and applying the predictor-corrector scheme described above.
and the molar volume v in both
phases and applying the predictor-corrector scheme described above.