Unlike biological colloids such as viruses, proteins and blood cells, synthetic colloids are never perfectly monodisperse. The influence of this polydispersity on the phase behavior of colloidal dispersions is not well understood. In the next chapter we discuss the effect of polydispersity on the freezing of hard spheres. In this section we combine the methods of this and the next chapter to assess the influence of polydispersity on the solid-solid transition.
As will be explained in detail in the next chapter, a simulation in the semigrand ensemble is done at constant N,V,T and 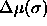 . Here
. Here 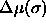 is the chemical potential
difference between a particle of diameter
is the chemical potential
difference between a particle of diameter 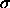 , and an arbitrarily
chosen reference component
, and an arbitrarily
chosen reference component 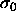 and is given by
and is given by
Sampling the diameters of the spheres according to this chemical potential gives rise to a Gaussian activity distribution that peaks at
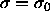 , with width
, with width  . For small
. For small  , the size composition
will also be Gaussian with the
peak near
, the size composition
will also be Gaussian with the
peak near 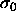 and the width of this distribution is
defined as the measure for size polydispersity s.
and the width of this distribution is
defined as the measure for size polydispersity s.
Using the same procedure as in the monodisperse case,
we performed semigrand simulations for a 108 sphere system with a
square well attraction in an fcc-configuration and
measured the internal energy.
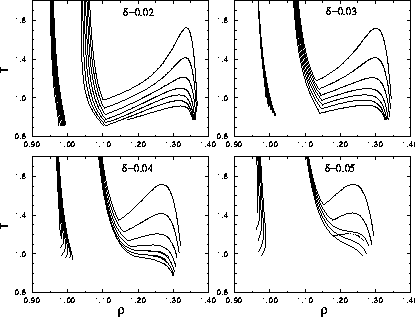
The simulations were done for  =0.02, 0.03, 0.04 and 0.05.
The polydispersity ranged from
=0.02, 0.03, 0.04 and 0.05.
The polydispersity ranged from 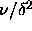 =0 to
=0 to
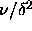 =0.1.
For every (
=0.1.
For every (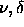 ) a complete set of densities was simulated for a range of temperatures.
The free energy was calculated using eqn.
3.2. Coexistence
was obtained by applying the common tangent construction.
) a complete set of densities was simulated for a range of temperatures.
The free energy was calculated using eqn.
3.2. Coexistence
was obtained by applying the common tangent construction.
Figure 3.11 shows the dependence of the polydispersity s for different  . Note that the critical temperature drops down as s increases.
Table 3.3 shows the maximum value of s beyond which the
solid-solid transition does not exist anymore. It appears to be
essentially independent of
. Note that the critical temperature drops down as s increases.
Table 3.3 shows the maximum value of s beyond which the
solid-solid transition does not exist anymore. It appears to be
essentially independent of  .
.
| Table: | Value of polydispersity at which solid-solid coexistence is preempted by melting transition. |