Figure 3.1 and 3.2
show the computed solid-solid and fluid-solid coexistence curves
in the 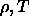 plane for the two and three dimensional square-well models.
We first focus on the solid-solid transition.
The density gap between the dense and expanded fcc solids
is wide at low temperatures and shrinks to zero when the solid-solid
critical point is approached.
Because of the analogy with liquid-vapor coexistence, one would expect that
the solid-solid critical point should be of the 2D and 3D-Ising universality
class.
plane for the two and three dimensional square-well models.
We first focus on the solid-solid transition.
The density gap between the dense and expanded fcc solids
is wide at low temperatures and shrinks to zero when the solid-solid
critical point is approached.
Because of the analogy with liquid-vapor coexistence, one would expect that
the solid-solid critical point should be of the 2D and 3D-Ising universality
class.
The coexistence curves are asymmetric, especially in the limit
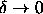 .
In this limit, the reduced critical temperature
.
In this limit, the reduced critical temperature 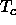 goes to a finite
limiting value of
approximately 1.7 for D=3 and 0.92 for D=2.
As we shall argue below, the phase behavior in this limit can be
investigated by studying a peculiar lattice model.
goes to a finite
limiting value of
approximately 1.7 for D=3 and 0.92 for D=2.
As we shall argue below, the phase behavior in this limit can be
investigated by studying a peculiar lattice model.
As can be seen in figure 3.1 and 3.2, the
critical temperature depends
only weakly on  . In contrast, the solid-solid coexistence region
shifts to lower densities as the well-width is increased. This effect
can easily be understood
by noting that a dense square-well solid can be expanded at virtually
no cost in
potential energy, up to the point where the nearest-neighbor separation is
1+
. In contrast, the solid-solid coexistence region
shifts to lower densities as the well-width is increased. This effect
can easily be understood
by noting that a dense square-well solid can be expanded at virtually
no cost in
potential energy, up to the point where the nearest-neighbor separation is
1+ , that is
, that is 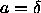 . It is only when the solid is
expanded beyond this limit that
the potential energy increases steeply and a transition to the expanded
solid may occur. Hence, the larger
. It is only when the solid is
expanded beyond this limit that
the potential energy increases steeply and a transition to the expanded
solid may occur. Hence, the larger  , the lower the density where the
phase transition will take place.
, the lower the density where the
phase transition will take place.
The fluid-solid coexistence curves are also dependent on  . For
small
. For
small  the width of the coexistence density gap between fluid
and solid remains effectively constant as a function of temperature,
although as a whole it shifts to higher density as the temperature is
lowered.
If
the width of the coexistence density gap between fluid
and solid remains effectively constant as a function of temperature,
although as a whole it shifts to higher density as the temperature is
lowered.
If  is increased the density gap widens at low temperature.
is increased the density gap widens at low temperature.
The point where the coexistence curves cross the solid-solid binodals
is the triple point 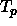 where the three phases (fluid,solid I and
solid II) are in equilibrium.
At temperatures below the triple point the high-density solid is in
equilibrium with a dilute gas.
When
where the three phases (fluid,solid I and
solid II) are in equilibrium.
At temperatures below the triple point the high-density solid is in
equilibrium with a dilute gas.
When  becomes larger, the triple point shifts to higher
temperatures and densities, until it
reaches the critical temperature. At that point the solid-solid
transition disappears because for larger values of
becomes larger, the triple point shifts to higher
temperatures and densities, until it
reaches the critical temperature. At that point the solid-solid
transition disappears because for larger values of  it is
preempted by the melting transition. Both in two and three
dimensions, this happens when
it is
preempted by the melting transition. Both in two and three
dimensions, this happens when 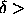 0.06.
0.06.
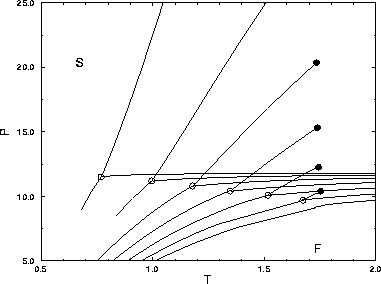
It is instructive to draw the phase diagram in the P,T plane. In
figure 3.3 the phase diagrams for the three dimensional
square well system are shown in the P,T plane. The solid-solid
transition lines run from the triple point to the critical point and
lie above the melting lines of the square well system. The phase
diagram for short-ranged attractive potential is the mirror image of
the usual P,T phase diagram for longer ranged potentials in which
the liquid-gas transition is present. Note that the slope of the
solid-solid transition line cannot be negative, because the critical
temperature has to be higher than the triple point temperature.