Gallery
- MPEG movie (1.2Mb) of
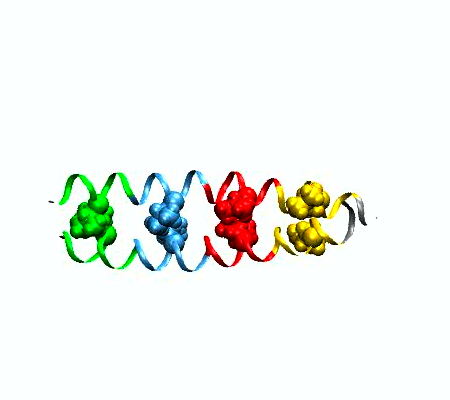
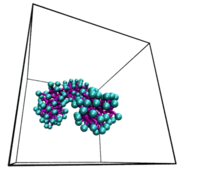
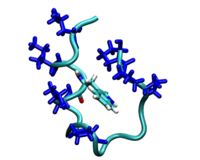
a protein dimer "unzipping". The leucine zipper
complex dissociates by applying adaptive potentials at
both ends of the complex. The colors indicate the four
heptad repeats, the structural units that are typical
for coiled coil complexes, comprising seven amino
acids. Leucine sidechains are depicted in spacefilling
representation. This work is part of the Veni
research project done by Jocelyne Vreede.
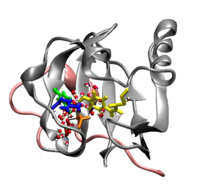
- MPEG movie (7Mb)
of the formation of the signaling state of Photoactive
Yellow Protein, obtained from parallel tempering
simulations. The hydrogen bond network around the
chromophore (yellow sticks) shifts towards Glutamic
acid 46 (red sticks), followed by hydration of the
chromophore binding pocket. Finally, the chromophore
becomes fully exposed to solvent. This research was
done by Jocelyne
Vreede. Interested in reading more? Click
here...
-
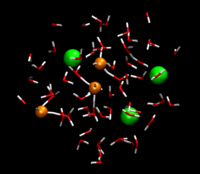
Jasper Heuft
studied in a systematic manner the aqueous solvent
structure around dissolved ions. This MPEG movie (60Mb!) obtained from an
ab initio molecular dynamics simulation, shows the
microscopic behavior of hydrochloric acid in
water. Chloride ions are shown as green spheres and
water molecules are represented as red (oxygen) and
white (hydrogen) sticks. Four protons ride the
hydrogen bonded network and jump from H2O
to H2O. They light up as orange spheres
when they temporary form a "stable" hydronium complex.
- MPEG movie (6Mb)
It is well-established that micelle formation proceeds
via a nucleation mechanism. Recently, René Pool found
that a specific soap molecule enables another
mechanism for the formation of a micelle
solution. This replication mechanism involves
growth where the
cluster changes from a spherical to an elipsoidal
shape. A critical fluctuation to a dumbbell shape with
a narrow neck then leads to breakup into two daughter
micelles.
- MPEG movie (13Mb).
In this movie by Jarek
Juraszek, the folding of a small protein,
Trp-cage, is shown. Using transition path sampling,
the extended chain of amino acids collapses.
-
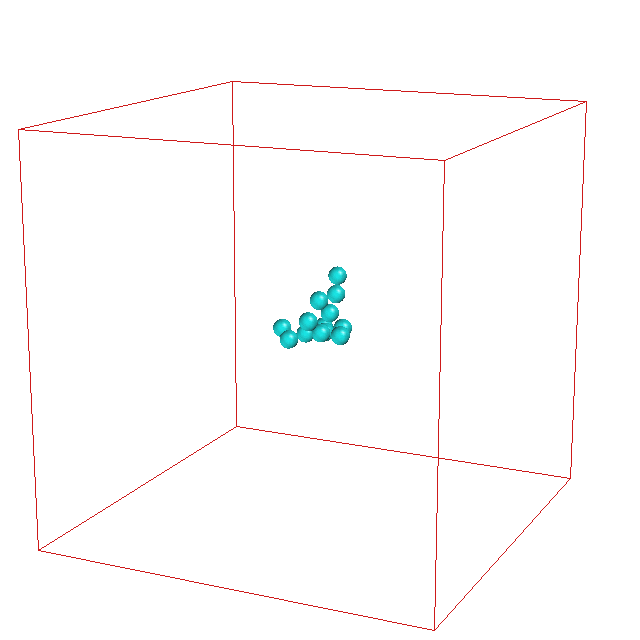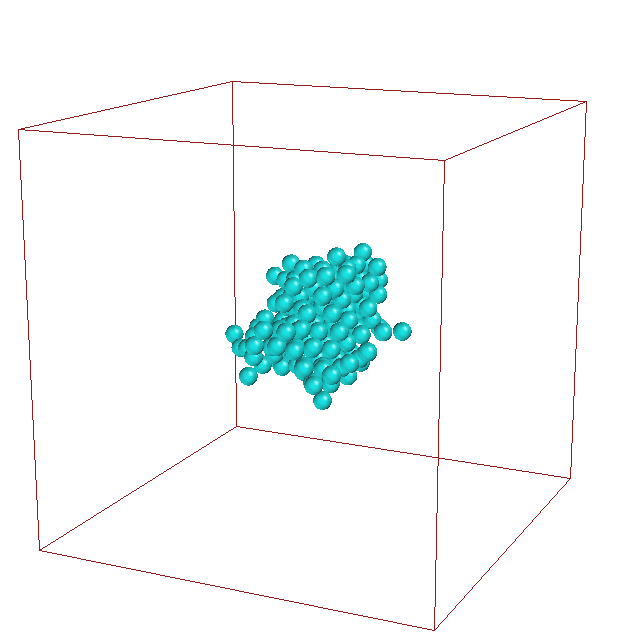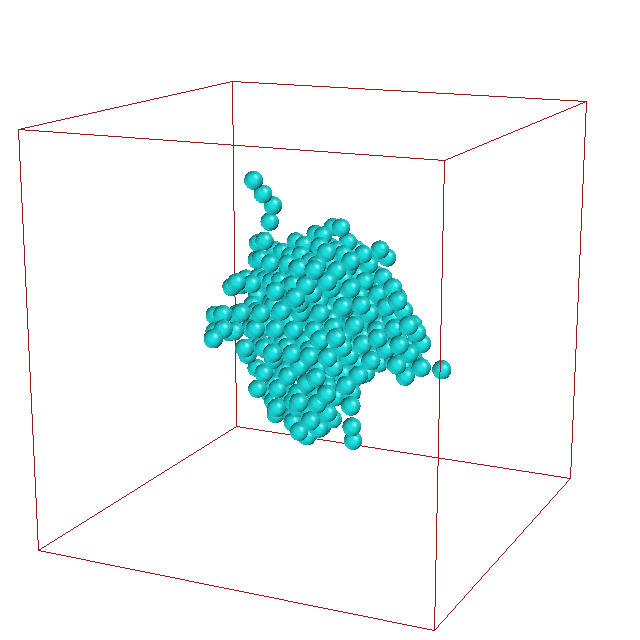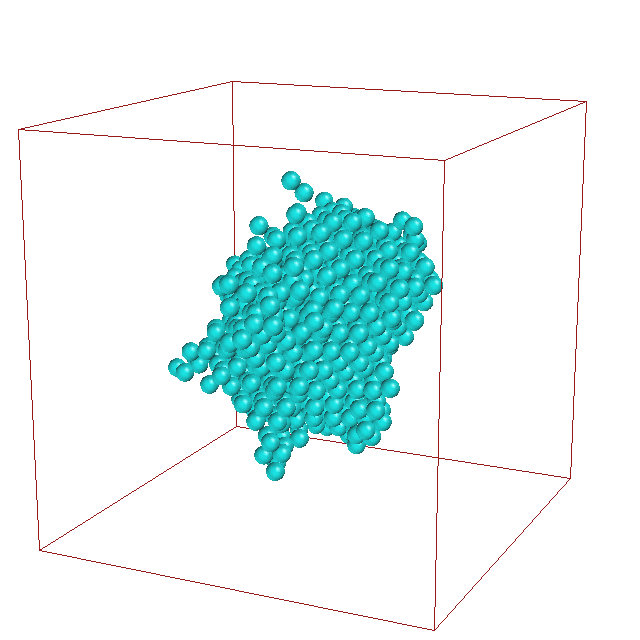
Daniele
Moroni studied the kinetics of crystal nucleation
of an undercooled Lennard-Jones liquid using various
path sampling methods. He obtained the rate constant
and elucidated the pathways for this nucleation
process. Analysis of the path ensemble revealed that
crystal nucleation occurs along many different
pathways, in which critical solid nuclei can be small,
compact, and face-centered-cubic, but also large, less
ordered, and more body- centered- cubic.
The fluctuations in the cluster shape are clearly visible in
this animation of a typical nucleation pathway.
-
This work was published as
The interplay between size and structure in the
critical nucleus,
D. Moroni, P.R. ten Wolde and P.G. Bolhuis, in
Phys. Rev. Lett. 94,
235703 (2005).
- AVI movie (16Mb)
of the ruthenium catalyzed hydrogen transfer of
formaldehyde to methanol in an explicit solvent model.
Jan-Willem
Handgraaf found that during the catalytic
conversion from the ketone to the corresponding
alcohol the solvent molecules actively participate in
the reaction. In the movie the reacting molecules are
shown in ball-stick representation. Green, red, blue,
cyan and white indicate ruthenium, oxygen, nitrogen,
carbon and hydrogen nuclei, respectively. Hydrogen
bonds are indicated by yellow dotted lines.
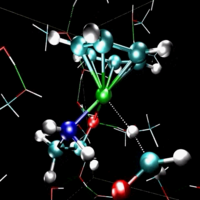
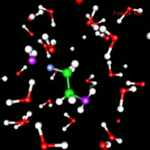
- MPEG movie (13Mb)
of water and ethene reacting to form ethanol. This
reaction is acid-catalysed, so the simulation box
contains an hydronium
ion (purple, left of the green ethene molecule). It
donates the blue proton to ethene and thereby giving
it a positive charge. The positive carbon of ethene
attracts the electronegative oxygen of the other purple water
molecule. When the oxygen attaches to ethene, it loses
one of its protons to another water molecule: Ethene
is transformed into ethanol and the hydronium ion is
recovered. This research was done by Titus van Erp.
- AVI movie with sound
(39Mb!) illustrating the high level of
experimental work done in the computational
chemistry group. The translation of the dialog between
the first
and the second
scientist is: " Ah, there forms a crystal, there
forms a crystal!" (1) and "it becomes
solid!" (2). The Parrinello group
is gratefully acknowledged for donating the equipment
(i.e. the chocolate fondu fountain).