chapter 1
 section 1.9
section 1.9
Many colloidal particles are non-spherical.
For instance, clay consists of
platelets, red blood cells are toroidal and other
colloids have a rodlike shape.
Examples of
colloidal rods are TMV and fd viruses, inorganic 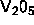 -particles
and boehmite needles. Rodlike particles have a richer phase diagram
than spherical colloids, because the rods can form a variety of
liquid-crystalline phases [24]. The term ``liquid crystal'' refers to a
structure that
is in between a crystalline solid and an isotropic liquid. Only
sufficiently non-spherical objects can form liquid
crystals.
-particles
and boehmite needles. Rodlike particles have a richer phase diagram
than spherical colloids, because the rods can form a variety of
liquid-crystalline phases [24]. The term ``liquid crystal'' refers to a
structure that
is in between a crystalline solid and an isotropic liquid. Only
sufficiently non-spherical objects can form liquid
crystals.
Already in the 1940's, Onsager showed that an isotropic dispersion of hard,
infinitely long rods, must undergo a first order orientational
ordering transition at sufficiently high
density [25]. The resulting nematic
liquid-crystalline phase is
characterized by long-range orientational ordering of the
particles. There is, however, no translational ordering: i.e. there
are no long-range positional correlations of the centers of mass of the
particles.
When compressed further, the system of hard rods can transform into
another liquid crystalline phase, called smectic. This phase has not only
orientational ordering but it also forms layers, in which the
particles are still free to move. There is only long-range
positional order in
one direction. Eventually, at sufficiently high pressures, crystallization
will take place, with long-range positional order in all three directions.
We show the sequence of liquid crystalline ordering in figure
1.4 for the case of a simple model of rodlike
particles, namely hard spherocylinders.
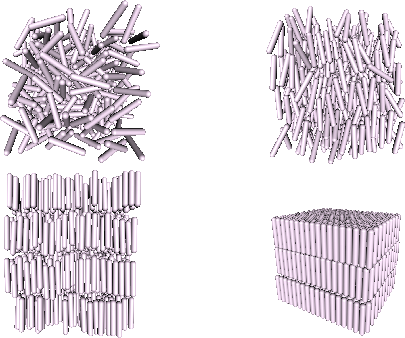
| Figure: | Sequence of phases of a system of hard
spherocylinder for increasing density. Top left: isotropic phase, no
translational and orientational ordering. Top
right: nematic liquid crystal, orientational ordering but no
translational. Bottom left: smectic liquid crystal, with besides
orientational ordering, translational ordering in the
z-direction. Bottom right: crystal phase |
Non-adsorbing polymers will also induce depletion interactions between
non-spherical colloids.
In fact, the addition of polymer can have a large effect on the
stability of the liquid crystalline
phases in dispersions of rodlike colloids.
The phase behavior of mixtures of
spherocylinder and polymers is one of the issues that will be
addressed in this thesis.
chapter 1
 section 1.9
section 1.9
Peter Bolhuis
Tue Sep 24 20:44:02 MDT 1996
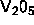 -particles
and boehmite needles. Rodlike particles have a richer phase diagram
than spherical colloids, because the rods can form a variety of
liquid-crystalline phases [24]. The term ``liquid crystal'' refers to a
structure that
is in between a crystalline solid and an isotropic liquid. Only
sufficiently non-spherical objects can form liquid
crystals.
-particles
and boehmite needles. Rodlike particles have a richer phase diagram
than spherical colloids, because the rods can form a variety of
liquid-crystalline phases [24]. The term ``liquid crystal'' refers to a
structure that
is in between a crystalline solid and an isotropic liquid. Only
sufficiently non-spherical objects can form liquid
crystals.
