Prof. dr. Peter Bolhuis
Van 't Hoff Institute for Molecular Sciences
University of Amsterdam
Science Park 904
PO Box 94157
1090 GD Amsterdam, The Netherlands
room: C2.222
phone: (+31) 20 525 6447
p.g.bolhuis (at) uva.nl
- Research Interests
- Publications
- Curriculum Vitae
- TPS Tutorial
Research in the Bolhuis Group
Our group is interested in studying rare events in complex systems such as folding of proteins, biomolecular isomerisation, self-assembly and nucleation events. To achieve insight in these processes we conduct multiscale modeling simulations of biomolecular systems using rare event- and coarse graining techniques. In addition we continue developing new and advanced simulation techniques.
A selection of current projects
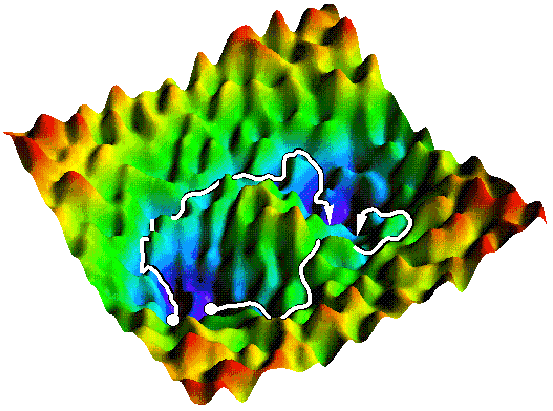
Transition path sampling methods
Transtion path sampling (TPS) was developed to harvest an ensemble unbiased dynamical reactive pathways. In the past years we have contributed to the development of the TPS framework, for instance, by further developing the Transition interface sampling to compute rate constants, and optimizing reaction coordinates. All of this culminated in the development of the OpenPathSampling python package.
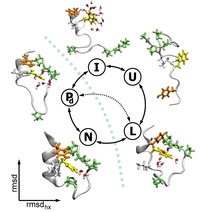
Kinetic Folding pathways of small proteins
Predicting the folding of proteins is still a major challenge in biologically inspired science. We sample unbiased folding pathways of small model proteins such as Trpcage and FBP28 WW domain using TPS in order to understand the folding pathways in atomistic detail. This leads to improved insight in the mechanism of folding and allows for accurate prediction of folding rates. Currently we study the influence of confinement on the folding pathways.
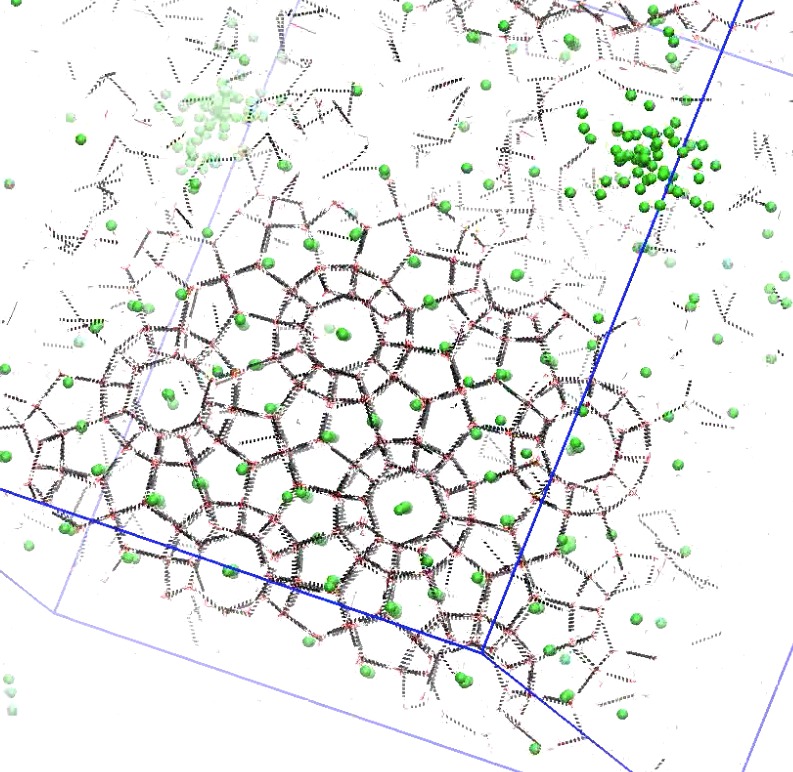
Nucleation of methane hydrate
Methane hydrates form spontaneously in methane/water mixtures under high pressure. The mechanism of formation by homogenous nucleation at moderate temperatures is still an open question. Previous simulation work necessarily focused on high undercooling conditions, or used coarse-grained water models that result in amorphous solid states instead of the experimentally found crystalline sI structure. This has led to multiple nucleation mechanism hypotheses. We apply Transition Path Sampling to elucidate the nucleation mechanism of methane hydrates at moderate conditions, and temperatures close to experimental values.

