By Francesco Colonna
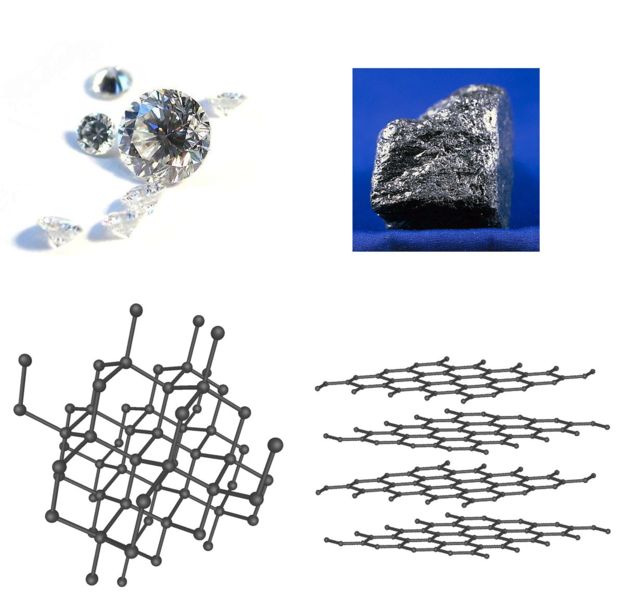
Melting and high temperature properties of graphite
Graphite is a carbon material with important technological applications. Graphite-like nanostructures, such as nanotubes and fullerens, are the basic building blocks of the nanotechnology. To improve the design and the synthesis of graphite-related materials, it is fundamental to have a good understanding of the high-temperature high-pressure behavior of graphite.
We studied the thermodynamics of graphite up to very high pressures (20GPa) and temperatures (4250K), as well as the structural and dynamical properties of liquid carbon close to the melting point of graphite.
Publications:
Properties of graphite at melting from multilayer thermodynamic integration - Phys. Rev. B 80, 134103 (2009)
"High-pressure high-temperature equation of state of graphite from Monte Carlo simulations" - Submitted
Stability and transformations of carbon nanotubes bundles
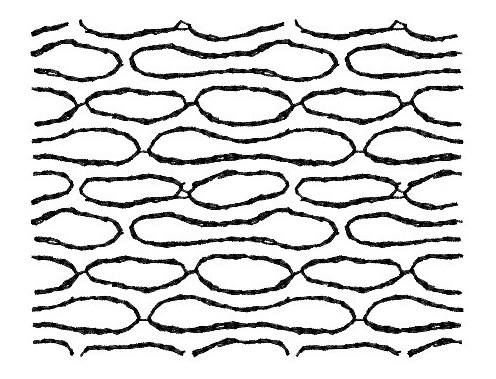 Carbon
nanotubes may self-assemble in bundles. At high pressure
these bundles undergo a structural phase transition. The tubes
may deform, collapse, or interlink depending on the pressure
and on the nature of the sample. When a high temperature is
applied in addition to pressure, the scenario is even more
complex: nanotubes can merge with each other, and the bundle
can transform into a different carbon structure. The reaction
diagram for the nanotubes is still unknown, and the
understanding of the transformation mechanisms is still at a
speculative stage.
Carbon
nanotubes may self-assemble in bundles. At high pressure
these bundles undergo a structural phase transition. The tubes
may deform, collapse, or interlink depending on the pressure
and on the nature of the sample. When a high temperature is
applied in addition to pressure, the scenario is even more
complex: nanotubes can merge with each other, and the bundle
can transform into a different carbon structure. The reaction
diagram for the nanotubes is still unknown, and the
understanding of the transformation mechanisms is still at a
speculative stage.
We are investigating the reaction diagram of singlewalled and multiwalled carbon nanotubes, as well as the mechanisms that lead to the formation of graphite and other related materials.
Super-hard materials at high temperature: the carbon clathrate
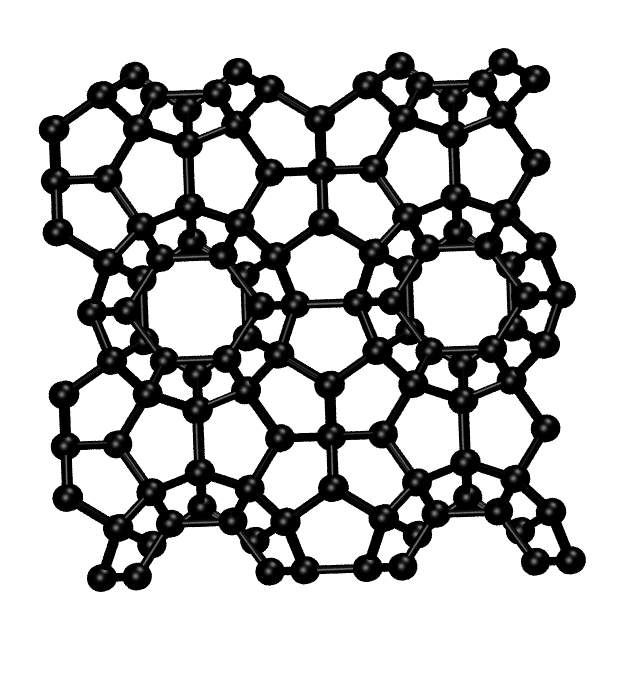 Clathrates
are an interesting family of solids with a cage-like
structure. At present, the carbon clathrate is still a
hypothetical material that experimentalist are trying to
synthesize. Ab initio simulations show that at very low
temperatures carbon clathrate is a super-hard superconducting
material with a great potential for technological
applications.
Clathrates
are an interesting family of solids with a cage-like
structure. At present, the carbon clathrate is still a
hypothetical material that experimentalist are trying to
synthesize. Ab initio simulations show that at very low
temperatures carbon clathrate is a super-hard superconducting
material with a great potential for technological
applications.
However, some important piece of information for the synthesis and usage of the carbon clathrate is still missing, like its structural stability and its thermodynamics at room and high temperature. We aim to provide these pieces of information by means of large-scale atomistic simulations.
About the possible melting of fullerite
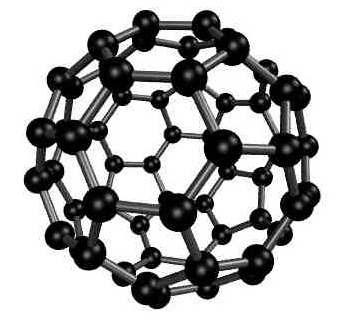 Fullerite is a molecular solid formed by fullerens. On
the basis of theoretical models, it has been predicted that at
moderate pressure the fullerite may melt into a fullerene
liquid, instead of sublimating into a fullerene
gas. Experiments, however, show that fullerite transforms into
a polymerized solid when the temperature is raised.
Fullerite is a molecular solid formed by fullerens. On
the basis of theoretical models, it has been predicted that at
moderate pressure the fullerite may melt into a fullerene
liquid, instead of sublimating into a fullerene
gas. Experiments, however, show that fullerite transforms into
a polymerized solid when the temperature is raised.
We
are investigating the origin of this discrepancy by means of
extensive atomistic simulations.