When water molecules meet air
By Ana Vila Verde
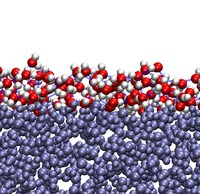
About 70% of our planet is covered in water. Most of that water exists as water in the bulk - the neighbors of water molecules are other water molecules - and only a small fraction of molecules are at the air-water interface. Despite the small relative abundance of interfacial water, it is of the utmost importance: it governs the chemistry involving the surface of oceans and seawater aerosols, or the small water droplets forming clouds. Ultimately, reactions at the air-water interface are directly relevant to world-scale phenomena such as the nutrient cycle in oceans or the evolution of the ozone layer. The air-water interface is also a good model for extended hydrophobic interfaces - the type of interface that occurs when water meets a large surface constituted by many apolar functional groups. Hydrophobic interfaces play a key role in the folding of proteins (the process by which proteins acquire their 3D structure) or the aggregation of surfactants.
Understanding many interfacial phenomena thus requires us to first understand the structure and dynamics of interfacial water. Studies of water at interfaces have only become possible within the past decades, with the advent of surface-sensitive experimental techniques such as vibrational sum-frequency (VSF) spectroscopy and the development of better classical models of water for molecular simulations. We have recently investigated the dynamics of a subpopulation of hydroxyl groups at the air-water interface: those OH groups that do not establish a hydrogen bond (called free OH groups)1. That population is very small in the bulk but much larger at the interface, and so is partially responsible for the interface's properties. Both classical molecular dynamics simulations (done in this group) and VSF experiments (done in Prof. Mischa Bonn's group) were used for this study. We found that the subpicosecond rotation of free OH groups is well described as a diffusive process, in strong contrast with rotation of hydrogen-bonded OH groups for water in the bulk, which proceeds mainly through large jumps. We also found that interfacial rotate several times faster than water in the bulk. These results will ultimately aid our understanding of the distinct kinetics of reactions taking place at the air-water interface.
Publications
Ultrafast Reorientation of Dangling OH Groups at the Air/Water Interface Physical review letters, 2011, 107, 116102
- Ultrafast Reorientation of Dangling OH Groups at the Air/Water Interface Phys. Rev. Lett. 107 (2011), 116102 DOI: 10.1103/PhysRevLett.107.116102