Dr. Ir. Bernd Ensing
Postal address:
Van 't Hoff Institute for Molecular Sciences
University of Amsterdam
PO Box 94157
1090 GD Amsterdam, The Netherlands
Visiting address:
Science Park 904
1098 XH Amsterdam
room: C2.238
phone: (+31) 20 525 5067
email: b.ensing uva.nl
uva.nl
Research Interests
Electron and proton transfer in solution
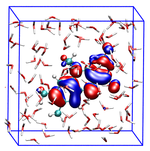
Protons and electrons are very light particles that can move with a high velocity, but in practice their transfer is strongly affected by the medium. The conductivity is governed by the solvent fluctuations. Confinement of the solvent in one or more dimensions affects the transfer mechanism. The solvent reorganization free energy is an important part of both the redox potential and the acidity constants of acceptor solutes.
Signal Transduction in Photoactive Proteins
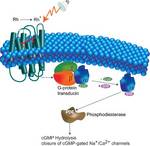
Photoactive proteins are light sensors that allow plants to turn their leafs toward the sun and allow animals (and people) to see. They form the perfect study ground to understand how (sensor) proteins work, because they can simply be activated with a flash of (laser) light, after which their functioning is probed.
Hybrid MD
We have developed a hybrid molecular dynamics method that is multiscale in both space and in time. This is particularly useful for large systems for which overall only a coarsegrained description is feasible. The hybrid MD method allows molecules to adapt their representation, between a fully atomistic and a coarsegrained resolution, on the fly in a smooth manner.
See also this research page or click here for an illustrative movie.
Finding the lowest free energy path
Chemical reactions are rare events on the picosecond time scale available to ab initio dynamics simulations. Using the metadynamics method we can nevertheless probe the multi-dimensional free energy landscape underlying (concerted) reactions. The lowest free energy path in this landscape provides important insight into the mechanism and rate of reactions.
Solvent effect on chemical reactions
PhD thesis
work with prof. E.J. Baerends
Solvent effects can have a very large influence
on the thermodynamics and the kinetics of chemical
reactions in water. We used Car-Parrinello molecular
dynamics simulations to study these effects
on prototypical organic reactions and
on certain transition metal catalyzed
oxidation reactions (Fenton chemistry).
A short lecture
A 15 minute seminar on molecular simulations of chemical reactions for the Exact Sciences Department (FNWI) of the University of Amsterdam on 2 October 2017.





