chapter 1
 section 1.4
section 1.4
A colloidal dispersion consist of microscopic solid (or liquid)
particles dispersed in a solvent [14]. The linear size of those particles
is typically between 1 nm and 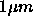 . Small enough to exhibit
Brownian motion caused by the surrounding solvent molecules but still
much larger than those molecules. Biological complexes like viruses,
proteins, micelles, vesicles consisting of membranes, blood cells but
also synthetic polymers belong to this class of particles. Colloids
are important in many industrial processes as well, as they occur in,
for example, paints, cosmetics and foods. During the past decades, much effort
has been devoted to the synthesis of well characterized colloidal
particles that have a very narrow distribution in sizes and
shapes. Such mono-disperse colloids can act as model systems to study
the factors that determine
the structure, dynamics and phase behavior of such systems [8]. There is a
surprising analogy between the statistical behavior of such colloidal
dispersions and that of simple atomic fluids. The statistical
thermodynamic properties can be derived in the same way as for atomic
systems, by treating the solvent as a continuous background that
exerts fluctuating forces on the colloidal particles. These Brownian
forces can be averaged and replaced by the so-called potential of mean
force. Onsager [15] and McMillan and Mayer
[16] showed that by using this effective potential as input
the full statistical mechanical machinery developed for atomic systems
can be used to describe the thermodynamic phase behavior of colloidal
dispersions.
. Small enough to exhibit
Brownian motion caused by the surrounding solvent molecules but still
much larger than those molecules. Biological complexes like viruses,
proteins, micelles, vesicles consisting of membranes, blood cells but
also synthetic polymers belong to this class of particles. Colloids
are important in many industrial processes as well, as they occur in,
for example, paints, cosmetics and foods. During the past decades, much effort
has been devoted to the synthesis of well characterized colloidal
particles that have a very narrow distribution in sizes and
shapes. Such mono-disperse colloids can act as model systems to study
the factors that determine
the structure, dynamics and phase behavior of such systems [8]. There is a
surprising analogy between the statistical behavior of such colloidal
dispersions and that of simple atomic fluids. The statistical
thermodynamic properties can be derived in the same way as for atomic
systems, by treating the solvent as a continuous background that
exerts fluctuating forces on the colloidal particles. These Brownian
forces can be averaged and replaced by the so-called potential of mean
force. Onsager [15] and McMillan and Mayer
[16] showed that by using this effective potential as input
the full statistical mechanical machinery developed for atomic systems
can be used to describe the thermodynamic phase behavior of colloidal
dispersions.
Peter Bolhuis
Tue Sep 24 20:44:02 MDT 1996
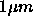 . Small enough to exhibit
Brownian motion caused by the surrounding solvent molecules but still
much larger than those molecules. Biological complexes like viruses,
proteins, micelles, vesicles consisting of membranes, blood cells but
also synthetic polymers belong to this class of particles. Colloids
are important in many industrial processes as well, as they occur in,
for example, paints, cosmetics and foods. During the past decades, much effort
has been devoted to the synthesis of well characterized colloidal
particles that have a very narrow distribution in sizes and
shapes. Such mono-disperse colloids can act as model systems to study
the factors that determine
the structure, dynamics and phase behavior of such systems [8]. There is a
surprising analogy between the statistical behavior of such colloidal
dispersions and that of simple atomic fluids. The statistical
thermodynamic properties can be derived in the same way as for atomic
systems, by treating the solvent as a continuous background that
exerts fluctuating forces on the colloidal particles. These Brownian
forces can be averaged and replaced by the so-called potential of mean
force. Onsager [15] and McMillan and Mayer
[16] showed that by using this effective potential as input
the full statistical mechanical machinery developed for atomic systems
can be used to describe the thermodynamic phase behavior of colloidal
dispersions.
. Small enough to exhibit
Brownian motion caused by the surrounding solvent molecules but still
much larger than those molecules. Biological complexes like viruses,
proteins, micelles, vesicles consisting of membranes, blood cells but
also synthetic polymers belong to this class of particles. Colloids
are important in many industrial processes as well, as they occur in,
for example, paints, cosmetics and foods. During the past decades, much effort
has been devoted to the synthesis of well characterized colloidal
particles that have a very narrow distribution in sizes and
shapes. Such mono-disperse colloids can act as model systems to study
the factors that determine
the structure, dynamics and phase behavior of such systems [8]. There is a
surprising analogy between the statistical behavior of such colloidal
dispersions and that of simple atomic fluids. The statistical
thermodynamic properties can be derived in the same way as for atomic
systems, by treating the solvent as a continuous background that
exerts fluctuating forces on the colloidal particles. These Brownian
forces can be averaged and replaced by the so-called potential of mean
force. Onsager [15] and McMillan and Mayer
[16] showed that by using this effective potential as input
the full statistical mechanical machinery developed for atomic systems
can be used to describe the thermodynamic phase behavior of colloidal
dispersions.