Metal Catalyzed Transfer Hydrogenation: The Role of the Solvent
By Anna Pavlova and Rosanne Zeiler
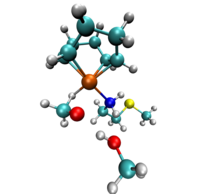
Hydrogenation of C=C, C=O and C=N bonds, resulting in alkanes, alcohols and amines, is one of the most essential transformations in chemistry. In a transfer hydrogenation reaction the reduction occurs by abstraction of hydrogens from a donor reagent followed by, or concerted with, addition of those hydrogens to the double bond of the substrate. With employment of transition metal catalysts highly selective and enantioselective reductions have been achieved, which is of great importance for pharmaceutical industry.
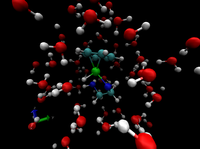
The choice of the solvent has been shown to have an immense effect on the reaction speed and enantioselectivity. The goal of our studies is to identify the different reaction pathways in solution for transfer hydrogenation with transition metal catalysts. We investigate hydrogen bonding and other possible solvent effects on the reaction barrier.
A realistic model of the studied system, requires the description of the electronic structure as well as the dynamics of the atoms. Car-Parinello Molecular Dynamics, CPMD, is an efficient method that combines Density Functional Theory with Molecular Dynamics and therefore is applied in our studies. Constrained CPMD simulations are employed in order to study the reaction profiles and the associated free energies.